| |
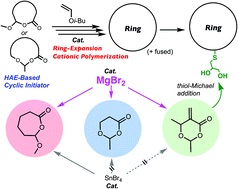
- "Magnesium bromide (MgBr2) as a catalyst for living cationic polymerization
and ring-expansion cationic polymerization"
Y. Daito, R. Kojima, N. Kusuyama, Y. Kohsaka, M. Ouchi, Polym. Chem. 2021, in press (DOI: 10.1039/D0PY01584A)
Collaboration with Prof. Ouchi's Group in Kyoto University; Invited for 'Molecularly Defined Polymers: Synthesis and Function'
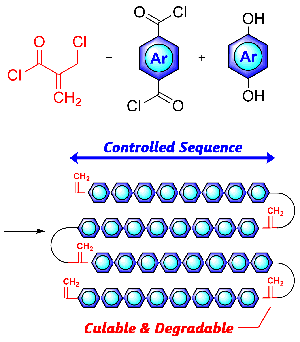
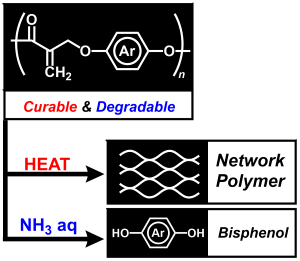
- "Controls and Effects of Monomer Junctions and Sequences in Curable
and Degradable Polyarylate Containing Acrylate Moieties"
Y. Kohsaka, K. Nagai, Macromol. Rapid Commun. 2021, in press (doi: 10.1002/marc.202000570) 
- "Degradable and curable poly(conjugated ester)s prepared by acryl-
and conjugate-substitutions of the ‘smallest’ monomer"
Y. Kohsaka, K. Nagai, Eur. Polym. J. 2020, 141, 110049.) 
- "Divergence of polycondensation by a tandem reaction based on sequential
conjugate substitutions"
K. Hagiwara, Y. Kohsaka, Polym. Chemistry. 2020, 11, 5128-5132. (Back Cover) 

- "End-reactive poly(tetrahydrofuran) for functionalization and graft
copolymer synthesis via a conjugate substitution reaction"
Y. Kohsaka, N. Nagatsuka, Polym. J. 2020, 52, 75 (doi: 10.1038/s41428-019-0258-4) 
- "Synthesis of Poly(Conjugated Ester)s by Ring-Opening Polymerization of Cyclic Hemiacetal Ester Bearing Acryl Skeleton"
Y. Kohsaka, M. Yamashita, Y. Matsuhashi, S. Yamashita, Eur. Polym. J. 2019, 120, 109185 
- "Synthesis and properties of polyethers containing 1,3-butadiene skeleton in the backbones"
Y. Kohsaka, A. Hiramatsu, Chem. Lett. 2019, 48, 894. 
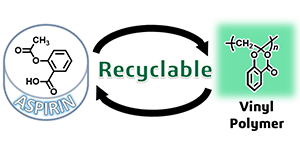
- "Radical Polymerization of ‘Dehydroaspirin’ with a Formation of Hemiacetal
Ester Skeleton: A Hint for Recyclable Vinyl Polymers"
A. Kazama, Y. Kohsaka, Polym. Chem. 10, 2764-2768 (2019) (10.1039/C9PY00474B) (Inside Cover). 

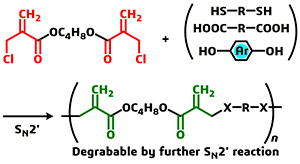
- "Conjugate substitution and addition of α-substituted acrylate: a
highly efficient, facile, convenient, and versatile approach to fabricate
degradable polymers "
Y. Kohsaka, T. Miyazaki, K. Hagiwara, Polym. Chem. 9, 1610-1617 (2018) (DOI:10.1039/C7PY02114C). (Invited as Emerging Innovators) 
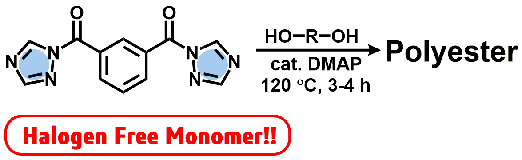
- "Bifunctional Acyl-1,2,4-triazole: An Alternative Monomer of Dicarbonyl
Chloride for Metal- and Halogen-Free Polyester Synthesis"
Y. Kohsaka, K. Homma, I. Mori, S. Sugiyama, Y. Kimura, Chem. Lett, 47, 221-224 (2018) .
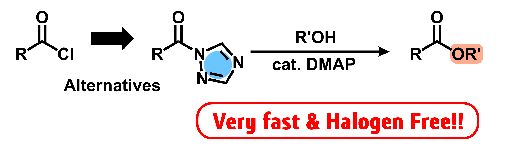
- "Esterification with aromatic acyl-1,2,4-triazole catalyzed by weak
base at the rate comparable to acyl chloride"
Y. Kohsaka, K. Homma, S. Sugiyama, Y. Kimura, Chem. Lett, 47, 100-102 (2018).
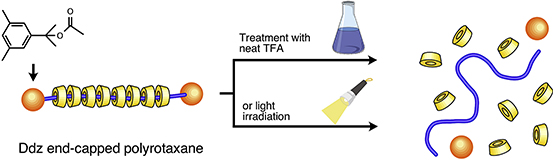
- "Acid- or photo-cleavable polyrotaxane: Subdivision of supramolecular main-chain type polyrotaxane structure induced by acidolysis or photolysis"
J. Araki, Y. Honda, Y. Kohsaka, Polymer, 25, 134-137 (2017).
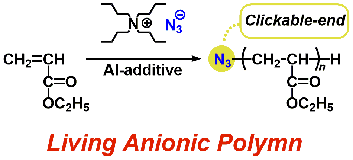
- "Anionic polymerization of ethyl acrylate initiated by tetrabutylammonium
azide: direct synthesis of end-clickable polyacrylate"
Y. Kataoka, Y. Kohsaka, T. Kitaura, S. Domae, S. Ishihara, T. Kitayama,
Polym. Chem. 8, 3858-3861 (2017). (Front Cover) 
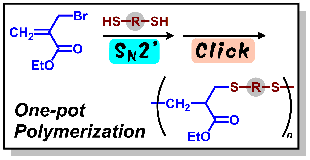
- "Polymerization of α-(Halomethyl)acrylates through Sequential Nucleophilic
Attack of Dithiols using a Combination of Addition–Elimination and Click
Reactions"
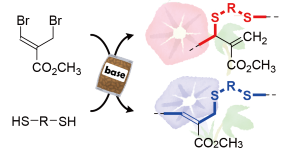
Y. Kohsaka, K. Hagiwara, K. Ito, Polym. Chem. 8, 976-979 (2017).
- "Synthesis of isotactic poly[α-(hydroxymethyl)acrylate] by anionic
polymerization of the protected monomer"
Y. Kohsaka, K. Yamamoto, K. Suzawa, T. Kitayama, Polym. Bull. 74, 1934-1948 (2017). 
- "Synthesis of thermo-responsive polymer via radical (co)polymerization of N,N-dimethyl-α-(hydroxymethyl)acrylamide with N,N-diethylacrylamide"
Y. Kohsaka, Y. Tanimoto, Polymers 2016, 8, 374 (Open Access).
- "Synthesis and reaction of block copolymer composed of independently
clickable segments via monomer-selective living copolymerization"
V. Ladelta, Y. Kohsaka, T. Kitayama, Microsystem Tech. in press. 
- "α-Exomethylene lactone possessing acetal-ester linkage: Polymerization
and postpolymerization modification for water-soluble polymer"
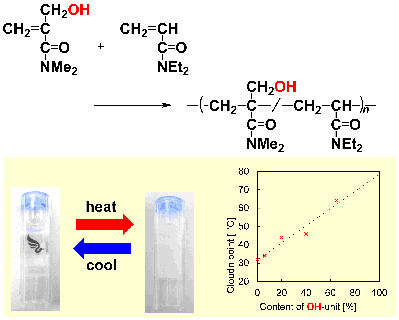
Y. Kohsaka, Y. Matsumoto, T. Zhang, Y. Matsuhashi, T. Kitayama, J. Polym. Sci. Part A: Polym. Chem. 54, 955-961 (2016). (Spotlight Article)
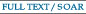
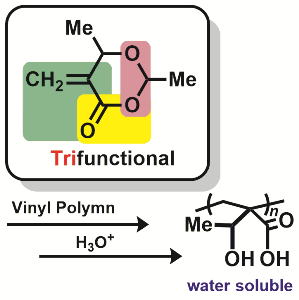
- "α-(Aminomethyl)acrylate: polymerization and spontaneous post-polymerization
modification of β-amino acid ester for a pH/temperature-responsive material
"
Y. Kohsaka, Y. Matsumoto, T. Kitayama, Polym. Chem.6, 5026-5029 (2015). 
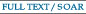
- "Termination of living anionic polymerization of butyl acrylate with
α-(chloromethyl)acrylate for end-functionalization and application to the
evaluation of monomer reactivity"
Y. Kohsaka, S. Ishihara, T. Kitayama, Macromol. Chem. Phys. 216, 1534-1539 (2015). 
- "Stereoregular poly(methyl methacrylate) with double-clickable ω-end: Synthesis and click reaction"
Y. Kohsaka, K. Yamamoto, T. Kitayama, Polym. Chem. 6, 3601-3607 (2015).  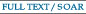 (Inside Cover) (Inside Cover)
- "C60-Containing polymethacrylates: synthesis, properties, and potential application
as n-type semiconductor for organic solar cell"
V. Ladelta, Y. Kohsaka, T. Ohsnishi, M. Matsumura, and T. Kitayama, Polym. Bull. 72, 1265-1280 (2015). 
- "Stereospecific Anionic Polymerization of α-(Hydroxymethyl)acrylate with Protective Group"
Y. Kohsaka, K. Suzawa, T. Kitayama, Macromol. Symp. 350, 86-98 (2015). 
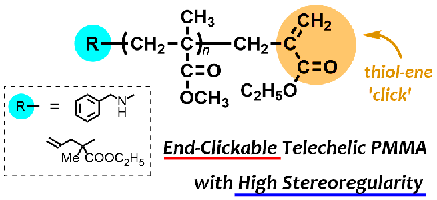
- "Synthesis and post-polymerization reaction of end-clickable stereoregular polymethacrylates via termination of stereospecific living anionic polymerization"
Y. Kohsaka, T. Kurata, K. Yamamoto, S. Ishihara, T. Kitayama, Polym. Chem. 6, 1078-1087 (2015). 
- “Anionic alternating copolymerization of alpha-arylacrylates with methyl
methacrylate: effect of monomer sequence on fluorescence”
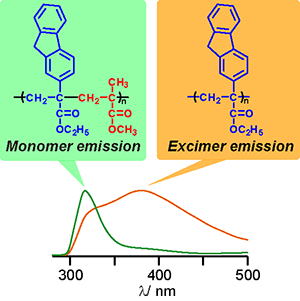
Y. Kohsaka, E. Yamaguchi, T. Kitayama, J. Polym. Sci. Part A: Polym. Chem. 52, 2806-2814 (2014). 
- “Stimuli-degradable cross-linked polymers synthesized by radical polymerization
using a size-complementary [3]rotaxane cross-linker”
K. Iijima, Y. Kohsaka, Y. Koyama, K. Nakazono, S. Uchida, S. Asai, T. Takata, Polym. J. 46, 67-72 (2014). 
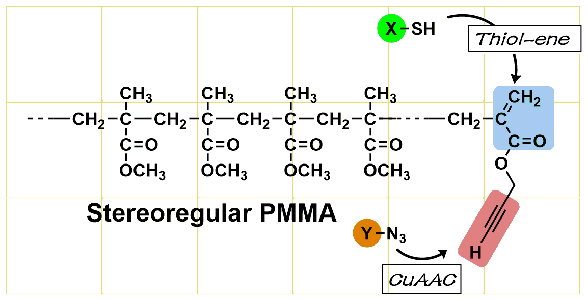
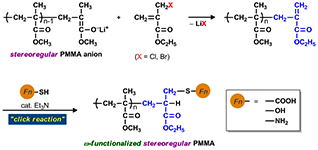
- “End-functional stereoregular poly(methyl methacrylate) with clickable C=C bonds: Facile synthesis and thiol?ene reaction”
Y. Kohsaka, T. Kurata, T. Kitayama, Polym. Chem. 4, 5043-5047 (2013). 
- “Photo-degradable cross-linked polymer derived from a vinylic rotaxane
cross-linker possessing aromatic disulfide axle”
Y. Koyama, T. Yoshii, Y. Kohsaka, T. Takata, Pure Appl. Chem. 85, 835-842 (2013). 
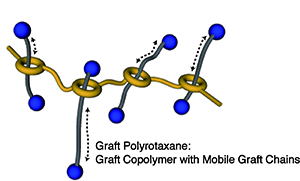
- “Graft polyrotaxane: A new class of graft copolymer with mobile graft chains”
Y. Kohsaka, Y. Koyama, T. Takata, Angew. Chem. Int. Ed. 50, 10417-10420 (2011).  (Introduced by Chemistry World Magazine 2011) (Introduced by Chemistry World Magazine 2011)
- “Size-complementary rotaxane cross-link for stabilization and degradation
of supramolecular network”
Y. Kohsaka, K. Nakazono, Y. Koyama, S. Asai, T. Takata, Angew. Chem. Int. Ed. 50, 4872-4875 (2011).  (Intoroduced by NPG Asia Materials 2011) (Intoroduced by NPG Asia Materials 2011)

- “An efficient synthetic entry to rotaxanes utilising reversible cleavage of aromatic disulphide bonds”
T. Yoshii, Y. Kohsaka, T. Moriyama, T. Suzuki, Y. Koyama, T. Takata, Supramol. Chem. 23, 65-68 (2011). 
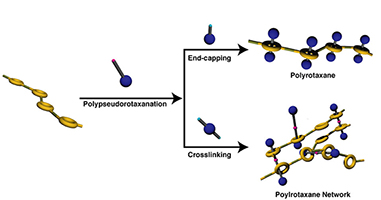
- “Synthesis of a main chain-type polyrotaxane consisting of poly(crown ether)
and dumbbell sec-ammonium salt and its application to polyrotaxane network”
Y. Kohsaka, G. Konishi, T. Takata, Polym. J. 39, 861-873 (2007). 
- “Main chain-type polyrotaxane with controlled ratio of rotaxanated units”
T. Takata, Y. Kohsaka, G. Konishi, Chem. Lett. 36, 292-293 (2007). 
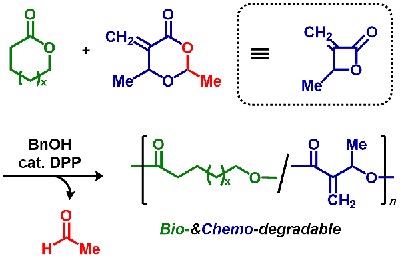
- "Degradable and curable poly(conjugated ester)s prepared by acryl-
and conjugate-substitutions of the ‘smallest’ monomer"
Y. Kohsaka, K. Nagai, Eur. Polym. J. 2020, in press (doi: 10.1016/j.eurpolymj.2020.110049) 

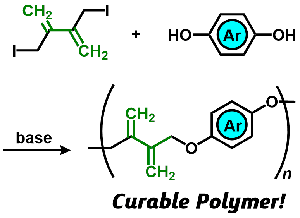
- "Divergence of polycondensation by a tandem reaction based on sequential
conjugate substitutions"
K. Hagiwara, Y. Kohsaka, Polym. Chemistry. 2020, in press (doi: 10.1039/D0PY00648C) 

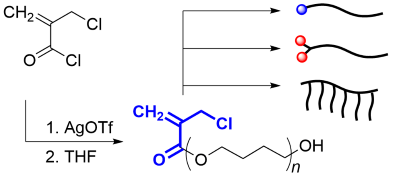
- "End-reactive poly(tetrahydrofuran) for functionalization and graft
copolymer synthesis via a conjugate substitution reaction"
Y. Kohsaka, N. Nagatsuka, Polym. J. in press (doi: 10.1038/s41428-019-0258-4) 

- "Synthesis of Poly(Conjugated Ester)s by Ring-Opening Polymerization of Cyclic Hemiacetal Ester Bearing Acryl Skeleton"
Y. Kohsaka, M. Yamashita, Y. Matsuhashi, S. Yamashita, Eur. Polym. J. in press (doi:/10.1016/j.eurpolymj.2019.08.012) 

- "Synthesis and properties of polyethers containing 1,3-butadiene skeleton in the backbones"
Y. Kohsaka, A. Hiramatsu, Chem. Lett. in press.(10.1246/cl.190381). 

- "Radical Polymerization of ‘Dehydroaspirin’ with a Formation of Hemiacetal
Ester Skeleton: A Hint for Recyclable Vinyl Polymers"
A. Kazama, Y. Kohsaka, Polym. Chem. 10, 2764-2768 (2019) (10.1039/C9PY00474B) (Inside Cover). 


- "Conjugate substitution and addition of α-substituted acrylate: a
highly efficient, facile, convenient, and versatile approach to fabricate
degradable polymers "
Y. Kohsaka, T. Miyazaki, K. Hagiwara, Polym. Chem. in press (DOI:10.1039/C7PY02114C). (Invited as Emerging Innovators) 

- "Bifunctional Acyl-1,2,4-triazole: An Alternative Monomer of Dicarbonyl
Chloride for Metal- and Halogen-Free Polyester Synthesis"
Y. Kohsaka, K. Homma, I. Mori, S. Sugiyama, Y. Kimura, Chem. Lett, acceptted.

- "Esterification with aromatic acyl-1,2,4-triazole catalyzed by weak
base at the rate comparable to acyl chloride"
Y. Kohsaka, K. Homma, S. Sugiyama, Y. Kimura, Chem. Lett, acceptted.

- "Acid- or photo-cleavable polyrotaxane: Subdivision of supramolecular main-chain type polyrotaxane structure induced by acidolysis or photolysis"
J. Araki, Y. Honda, Y. Kohsaka, Polymer, 25, 134-137 (2017).

- "Anionic polymerization of ethyl acrylate initiated by tetrabutylammonium
azide: direct synthesis of end-clickable polyacrylate"
Y. Kataoka, Y. Kohsaka, T. Kitaura, S. Domae, S. Ishihara, T. Kitayama,
Polym. Chem. 8, 3858-3861 (2017). (Front Cover) 

- "Polymerization of α-(Halomethyl)acrylates through Sequential Nucleophilic
Attack of Dithiols using a Combination of Addition–Elimination and Click
Reactions"
Y. Kohsaka, K. Hagiwara, K. Ito, Polym. Chem. 8, 976-979 (2017).

- "Synthesis of isotactic poly[α-(hydroxymethyl)acrylate] by anionic
polymerization of the protected monomer"
Y. Kohsaka, K. Yamamoto, K. Suzawa, T. Kitayama, Polym. Bull. 74, 1934-1948 (2017). 
- "Synthesis of thermo-responsive polymer via radical (co)polymerization of N,N-dimethyl-α-(hydroxymethyl)acrylamide with N,N-diethylacrylamide"
Y. Kohsaka, Y. Tanimoto, Polymers 2016, 8, 374 (Open Access).

- "Synthesis and reaction of block copolymer composed of independently
clickable segments via monomer-selective living copolymerization"
V. Ladelta, Y. Kohsaka, T. Kitayama, Microsystem Tech. in press. 
- "α-Exomethylene lactone possessing acetal-ester linkage: Polymerization
and postpolymerization modification for water-soluble polymer"
Y. Kohsaka, Y. Matsumoto, T. Zhang, Y. Matsuhashi, T. Kitayama, J. Polym. Sci. Part A: Polym. Chem. 54, 955-961 (2016). (Spotlight Article)

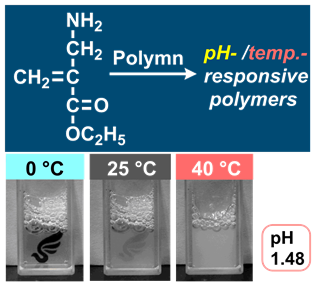
- "α-(Aminomethyl)acrylate: polymerization and spontaneous post-polymerization
modification of β-amino acid ester for a pH/temperature-responsive material
"
Y. Kohsaka, Y. Matsumoto, T. Kitayama, Polym. Chem.6, 5026-5029 (2015). 

- "Termination of living anionic polymerization of butyl acrylate with
α-(chloromethyl)acrylate for end-functionalization and application to the
evaluation of monomer reactivity"
Y. Kohsaka, S. Ishihara, T. Kitayama, Macromol. Chem. Phys. 216, 1534-1539 (2015). 
- "Stereoregular poly(methyl methacrylate) with double-clickable ω-end: Synthesis and click reaction"
Y. Kohsaka, K. Yamamoto, T. Kitayama, Polym. Chem. 6, 3601-3607 (2015).   (Inside Cover) (Inside Cover)

- "C60-Containing polymethacrylates: synthesis, properties, and potential application
as n-type semiconductor for organic solar cell"
V. Ladelta, Y. Kohsaka, T. Ohsnishi, M. Matsumura, and T. Kitayama, Polym. Bull. 72, 1265-1280 (2015). 
- "Stereospecific Anionic Polymerization of α-(Hydroxymethyl)acrylate with Protective Group"
Y. Kohsaka, K. Suzawa, T. Kitayama, Macromol. Symp. 350, 86-98 (2015). 

- "Synthesis and post-polymerization reaction of end-clickable stereoregular polymethacrylates via termination of stereospecific living anionic polymerization"
Y. Kohsaka, T. Kurata, K. Yamamoto, S. Ishihara, T. Kitayama, Polym. Chem. 6, 1078-1087 (2015). 

- “Anionic alternating copolymerization of alpha-arylacrylates with methyl
methacrylate: effect of monomer sequence on fluorescence”
Y. Kohsaka, E. Yamaguchi, T. Kitayama, J. Polym. Sci. Part A: Polym. Chem. 52, 2806-2814 (2014). 

- “Stimuli-degradable cross-linked polymers synthesized by radical polymerization
using a size-complementary [3]rotaxane cross-linker”
K. Iijima, Y. Kohsaka, Y. Koyama, K. Nakazono, S. Uchida, S. Asai, T. Takata, Polym. J. 46, 67-72 (2014). 
- “End-functional stereoregular poly(methyl methacrylate) with clickable C=C bonds: Facile synthesis and thiol?ene reaction”
Y. Kohsaka, T. Kurata, T. Kitayama, Polym. Chem. 4, 5043-5047 (2013). 

- “Photo-degradable cross-linked polymer derived from a vinylic rotaxane
cross-linker possessing aromatic disulfide axle”
Y. Koyama, T. Yoshii, Y. Kohsaka, T. Takata, Pure Appl. Chem. 85, 835-842 (2013). 
- “Graft polyrotaxane: A new class of graft copolymer with mobile graft chains”
Y. Kohsaka, Y. Koyama, T. Takata, Angew. Chem. Int. Ed. 50, 10417-10420 (2011).  (Introduced by Chemistry World Magazine 2011) (Introduced by Chemistry World Magazine 2011)

- “Size-complementary rotaxane cross-link for stabilization and degradation
of supramolecular network”
Y. Kohsaka, K. Nakazono, Y. Koyama, S. Asai, T. Takata, Angew. Chem. Int. Ed. 50, 4872-4875 (2011).  (Intoroduced by NPG Asia Materials 2011) (Intoroduced by NPG Asia Materials 2011)

- “An efficient synthetic entry to rotaxanes utilising reversible cleavage of aromatic disulphide bonds”
T. Yoshii, Y. Kohsaka, T. Moriyama, T. Suzuki, Y. Koyama, T. Takata, Supramol. Chem. 23, 65-68 (2011). 
- “Synthesis of a main chain-type polyrotaxane consisting of poly(crown ether)
and dumbbell sec-ammonium salt and its application to polyrotaxane network”
Y. Kohsaka, G. Konishi, T. Takata, Polym. J. 39, 861-873 (2007). 

- “Main chain-type polyrotaxane with controlled ratio of rotaxanated units”
T. Takata, Y. Kohsaka, G. Konishi, Chem. Lett. 36, 292-293 (2007). 
|
|

