|
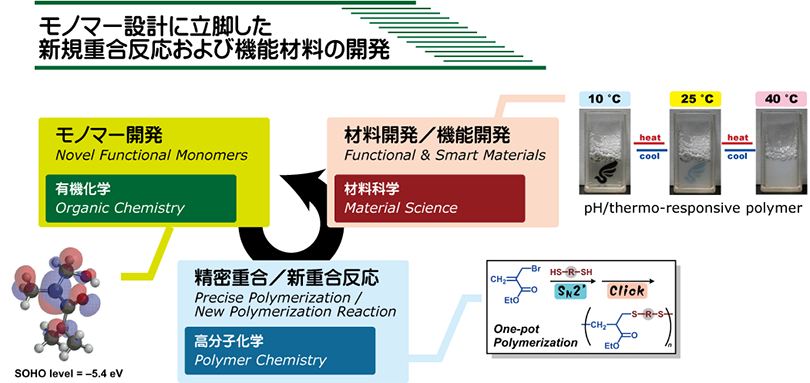
Macromolecular engineering is composed of three essences: (1) Monomer design,
(2) Polymerization design, and (3) Function design. However, these essences
are separated in each academic field, that is, organic chemistry, polymer
chemistry, and material science. In Kohsaka’s group, we recombine these
research fields and lead the total design of ‘new advanced polymer materials’
based on sophisticated (macro)molecular design.
|
|
|
|
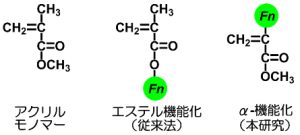
The major research interests are alpha-functionalized acryl monomer.
Acryl monomers are industrially important materials that are usually functionalized at the ester substituent. Functional group can be incorporated by a simple and facile reaction, esterification, while the functional group is far from the polymerization points so that they do not strongly affect the reaction.
In contrast, functionalization at the alpha-substituent is rare. The early
researches in 1950s-1960s have revealed that the substitution of alpha-position
with bulky group prevents the polymerization both in radical and anionic
mechanisms. Nowadays this is a ‘common sense’ and the functionalization
at the alpha-position seems unpractical.
|
|
|
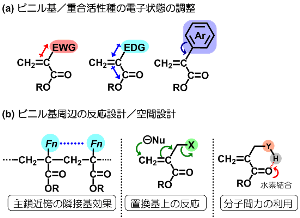
On the other hand, pioneering researches in 1970s-1990s suggest that some alpha-functionalized acrylates undergo polymerization in specific reaction mechanism. Inspired by them, we recognize alpha-functionalized acrylates as key materials leading novel polymerization and function.
Figures demonstrated in right means the strategy of our molecular design.
Here let me allow to avoid the detail as they include professional knowledges,
but we would emphasize that such alpha-functionalized acrylates provide
remarkable new function and/or reactivity, although they are very simple
molecules |
 |
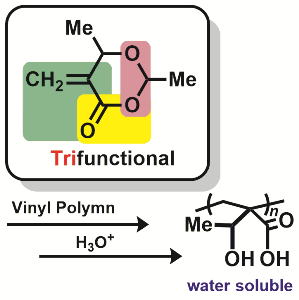
Polymerization of Highly Functionalized Ring
The 6-membered lactone described in right has three functional groups in
the ring, that is, vinyl group, ester bond, and acetal bond. As 5 of 6
atoms join the functions, the lactone can be regarded ultimately functionalized.
Vinyl polymerization can be employed with radical and anionic initiator,
while cationic and anionic ring-opening polymerization might be applicable.
Now we have proceeded the projects on the polymerization and post-polymerization
modification.
[Relative paper]
"α-Exomethylene lactone possessing acetal-ester linkage: Polymerization
and postpolymerization modification for water-soluble polymer"
Y. Kohsaka, Y. Matsumoto, T. Zhang, Y. Matsuhashi, T. Kitayama, J. Polym. Sci. Part A: Polym. Chem. 54, 955-961 (2016). (Spotlight Article)  
|
 |
pH- and Thermo-Responsive Vinyl Polymers
by Amino Acid Derivatives
Amino acids bearing polymerizable vinyl group are interesting platforms
to prepare stimuli-responsive biocompatible vinyl polymers. alpha-(Aminomethyl)acrylic
acid is a naturally occurring beta-amino acid. We have found the radical
polymerization of the monomer proceeds in a unique mechanism to afford
pH- and thermos-responsive polymer in acidic aqueous media. Further researches
focused on both polymerization chemistry and material science are under
construction.
[Relative paper]
"α-(Aminomethyl)acrylate: polymerization and spontaneous post-polymerization
modification of β-amino acid ester for a pH/temperature-responsive material
"
Y. Kohsaka, Y. Matsumoto, T. Kitayama, Polym. Chem. 6, 5026-5029 (2015).

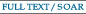

|
|
|
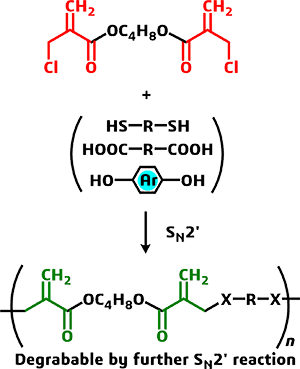
Synthesis of Chemically Degradable Biocompatible Polymer Based on Conjugate
Substituon
alpha-(Halomethyl)acrylates, which have characters of both acrylate and
allyl halide, undergoes conjugate substitution with various nucleophiles
under ambient conditions. The reaction products, alpha-(substituted methyl)acrylates,
are still active to conjugate substitution/addition. Therefore, we have
interested in the polycondensation of bis[alpha-(halomethyl)acrylates]
resulting in poly(conjugated esters), which can be decomposed by further
conjugate substitution. Similar poly(conjugated ester)s can be obtained
through the ring-opening polymerization of 6-membered cyclic hemiacetal
ester (lactone) described above.
[関連論文]
"Polymerization of α-(Halomethyl)acrylates through Sequential Nucleophilic
Attack of Dithiols using a Combination of Addition–Elimination and Click
Reactions"
Y. Kohsaka, K. Hagiwara, K. Ito, Polym. Chem. 8, 976-979 (2017).
"Conjugate substitution and addition of α-substituted acrylate: a highly efficient, facile, convenient, and versatile approach to fabricate degradable polymers by dynamic covalent chemistry"
Y. Kohsaka, T. Miyazaki, K. Hagiwara, Polym. Chem. advanced article.
DOI: 10.1039/C7PY02114C

 |
|
 |
|
| |
|
|

