| |
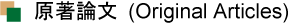
各論文のイメージ画像から,論文の出版社サイトにアクセスできます.
- "Vitrimer-like acrylic glass with fast stress relaxation by high-speed
carboxy exchange reaction""
Y. Kohsaka, M. Mizuma, M. Hayashi, RSC Appl. Polym. 2025. (Open Access)
https://doi.org/10.1039/D5LP00241A
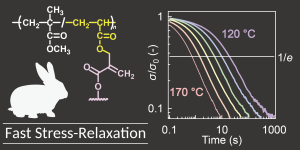
- "Poly(bridged bicycle)s synthesized via cyclopolymerization of cyclic
diacrylates"
Y. Kohsaka, T. Yoshida, N. Nishiie, Polym. Cem. 2025, 16, 4009-4012.
Pioneering Investigators 2025 特集号への招待論文
https://doi.org/10.1039/D5PY00631G
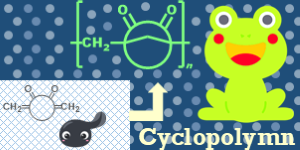 
- "Iterative Synthesis and Marine-Biodegradation Assessment of Discrete
Oligomers Based on Poly(Butylene Succinate), Poly(Ethylene Terephthalate),
Polyamide 6, and Polyisoprene"
T. Kubo, R. T. Ananthu, T. Noda, Y. Amamoto, H. Taguchi, Y. An, R. Kamiki, C. Homma, H. Masunaga, S. Sasaki, M. Uchiyama, M. Kamigaito, T. Kikuchi, Y. Kohsaka, A. Takahara, K. Satoh, Macromolecules 2025, 58 (16), 8649–8657 (Open Acess).
https://doi.org/10.1021/acs.macromol.5c01531
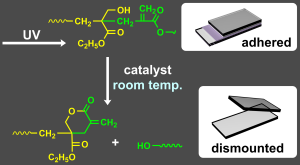
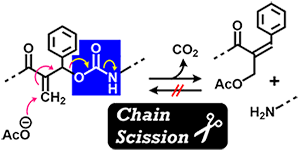
- "Main-Chain Scission of Acrylic Polymers via Intrachain Transesterification
and Their Application in UV-curable Dismountable Adhesives"
Y. Kohsaka, K. Naganuma, ACS Polyms Au 2025, 5, (5), 481–487(Open Acess).
https://doi.org/10.1021/acspolymersau.5c00060
- "Synthesis and polymerization of modified dehydroaspirin with increased
stability and polymer solubility"
Y. Kohsaka, H. Torisawa, A. Kazama, Polym. J. 2025, in press (Open Acess).
https://doi.org/10.1038/s41428-025-01070-4

- "Enhanced Recyclability of Methacrylic Resins by Copolymerization or Pendant Modification Using Trityl Esters"
Y. Chiba, S. Hirabayashi, Y. Kohsaka, Chemical Science 2025, 16, 12804-12811 (Open Access).
https://doi.org/10.1039/D5SC03190G


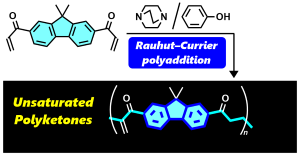
- "Rauhut–Currier Polyaddition: Self-Polymerization of Divalent Aryl
Vinyl Ketones for Unsaturated Polyketones"
M. Ohyama, R. Kawatani, N Ohtani, R. Fukuchi, R. Yasuda, H. Kuratani, S.
Miyauchi, Y. Kohsaka, Macromolecules 2025, 58, 5796-5806.
(Collaborated work with Osaka Gas Chemicals Co. Ltd.)
https://doi.org/10.1021/acs.macromol.5c00990
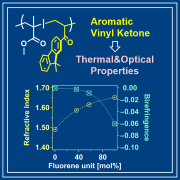
- "Vinyl Ketones Containing Fluorene Skeletons for Acrylic Resins with
High Refractive Index and Negative Birefringence"
M. Ohyama, R. Yasuda, H. Kuratani, S. Miyauchi, Y. Kohsaka, J. Polym. Sci. 2025, 63, 650-657.
(Collaborated work with Osaka Gas Chemicals Co. Ltd.)
https://doi.org/10.1002/pol.20240902 Open Access
- "Vitrimer-like Elastomers with Rapid Stress-Relaxation by High-Speed
Carboxy Exchange through Conjugate Substitution Reaction"
N. Nishiie, R. Kawatani, S. Tezuka, M. Mizuma, M. Hayashi, Y. Kohsaka,
Nat. Commun. 2024, 15, 8657.
(Collaborated work with Dr. M. Hayashi at NITech)
https://doi.org/10.1038/s41467-024-53043-5

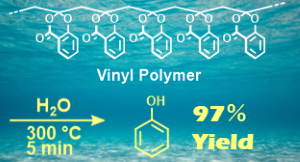
- "Carbon-Resource Recovery from Vinyl Polymers of Cyclic Ketene Acetal
Esters Using High-Temperature Water"
Y. Kohsaka, A. Kazama, K. Matsuo, S. Deguchi, M. Osada, ACS Sustain. Resour. Manage. 2024, 1, 2234-2240.
(Collaborated work with Prof. Deguchi at JAMSTEC and Prof. Osada at Shinshu
Univ.)
https://doi.org/10.1021/acssusresmgt.4c00260
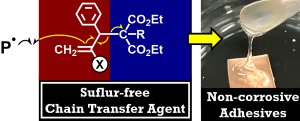
- "Noncorrosive Pressure-Sensitive Adhesives of Acryl Polymers by Sulfur-Free
Addition–Fragmentation Chain Transfer Agents"
R. Kawatani,Y. Mizuki, H. Matsuzaki, T. Miyamoto, Y. Kohsaka,Macromolecules 2024, 57, 8861.
Collaborated work with Soken Chemical & Engineering Co., Ltd.
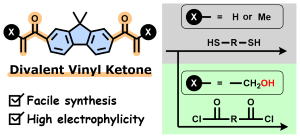
- "Divalent vinyl ketones derived from fluorene: a facile synthesis
of bifunctional acrylic monomers with high reactivity in thia-/aza-Michael
addition and Morita-Baylis-Hillman reactions"
M. Ohyama, R. Yasuda, S. Miyauchi, Y. Kohsaka,Polym. J. 2024, 56, 1111-1116.
Collaborated work with Osaka Gas Chemical Co., Ltd.
Open Access
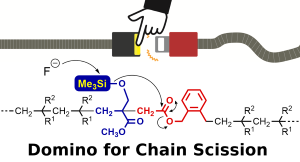
- "Fast and Selective Main-Chain Scission of Vinyl Polymers Using the
Domino Reaction in the Alternating Sequence for Transesterification"
Y. Kohsaka, K. Toyama, M. Kawauchi, K. Naganuma, ACS Macro Lett. 2024, 13, 1016-1021.
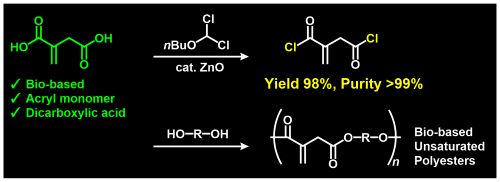
- "Synthesis of itaconyl dichloride with high purity and its application to polycondensation"
R. Kawatani, Y. Aoki, S. Tezuka, Y. Kimura, Y. Kohsaka, Tetradehron, 2024, 161, 134071.
(Collaborated work with Iharanikkei Chemical Industry Co. Ltd.)
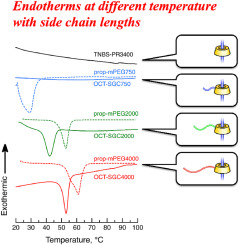
- "Regulatable endothermic behavior of one-chain-tethered sliding graft
copolymers as novel solid-solid phase change materials"
J. Araki, C. Otsubo, S. Morimoto, K. Ohta, Y. Kohsaka, Polymer, 2024, 294, 126687 (Collaborated work with Prof. Araki and Prof. Ohta)
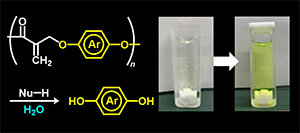
- "Degradation of poly(conjugated ester)s using a conjugate substitution
reaction with various amines and amino acids in aqueous media."
T. Noda, T. Kitagawa, Y. Kohsaka, Polym. J. 2024, 56, 343–35 (Invited for a special iIssue of Polymer Degradation for a Sustainable
Future)
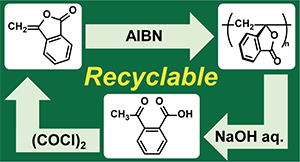
- "Chemically Recyclable Vinyl Polymers by Free Radical Polymerization of Cyclic Styrene Derivatives"
Y. Chiba, R. Kawatani, Y. Kohsaka, ACS Macro Lett. 2023, 12 (12), 1672–1676. (Ranked 2nd in Most Read papers in 30 days)

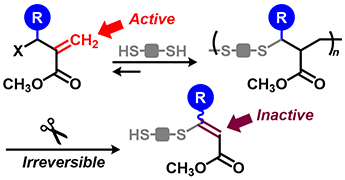
- "Polythioethers bearing side groups for efficient degradation by E1cB
reaction: reaction design for polymerization and main-chain scission"
R. Kawatani, K. Hagiwara, A. Tanaka, Y. Kohsaka, RSC Advances, 2023, 13, 20782-20786.
- "Unsaturated polyurethanes degradable by conjugate substitution reactions
with amines and carboxylate anions"
T. Noda, A. Tanaka, Y. Akae, Y. Kohsaka, RSC Adv. 2023, 13, 20336-20341.
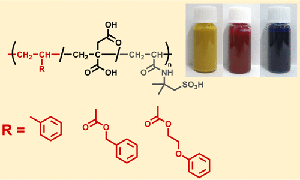
- "Polymeric Surfactants Bearing Divalent Carboxy Pendants for Stable Color Dispersions with High Redispersibility"
T. Ohtake, H. Ito, K. Horiba, N. Toyoda, Y. Kohsaka, ACS Appl. Mater. 2023, 5 (7), 4901–4909.
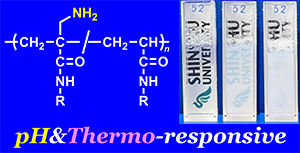
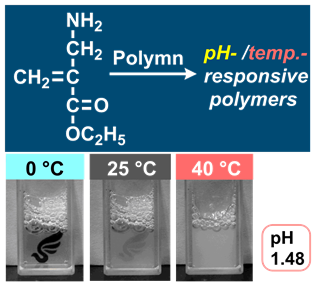
- "Effect of Amino Group Modification at Allyl Position of Methacrylamides
on Polymerization and Polymer pH-/Thermo-Responsiveness"
Y. Kohsaka, N. Chinbat, K. Ito, Y. Akae, Polym. Chem. 2023, 14,1585-1590.(Front Cover), (Invited Article)
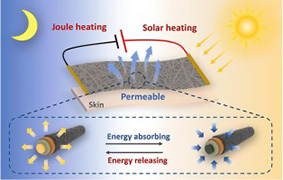
- "Highly integrated, breathable, metalized phase change fibrous membranes based on hierarchical coaxial fiber structure for multimodal personal thermal management"
J. Wu, M. Wang, L. Dong, Y. Zhang, J. Shi, M. Ohyama, Y. Kohsak, C. Zhu,
H. Morikawa, Chem. Eng. J. 2023, 465, 142835.
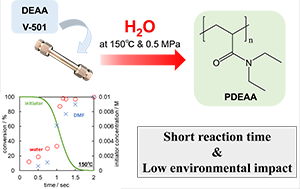
- High-Speed Synthesis of Thermo-responsive Polymers by Boosted Polymerization of N,N-Diethyl Acrylamide in High-Temperature Water
S. Ishii, A.i Sezai, Y. Kohsaka, S. Deguchi, and M. Osada*
Ind. Eng. Chem. Res. 2022, 61, 46, 17012–17016
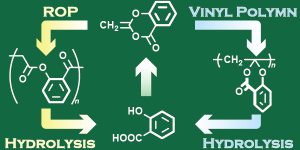
- Diverse chemically recyclable polymers obtained by cationic vinyl and ring-opening
polymerizations of the cyclic ketene acetal ester “dehydroaspirin”
A. Kazama, Y. Kohsaka, Polym. Chem. 2022,13, 6484-6491 (Back Cover)
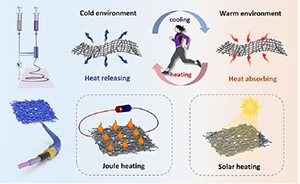
- A Trimode Thermoregulatory Flexible Fibrous Membrane Designed with Hierarchical Core–Sheath Fiber Structure for Wearable Personal Thermal Management
J. Wu, M. Wang, L. Dong, J. Shi, M. Ohyama, Y. Kohsaka, C. Zhu, H. Morikawa
ACS Nano, 2022, 16, 8, 12801–12812
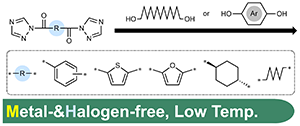
- Synthesis of polyarylates and aliphatic polyesters by divalent acyl-1,2,4-triazole:
a route to metal-free synthesis at low temperature
Y. Kohsaka, I. Mori, K. Homma, Y. Akae, D. Matsuura, Y. Kimura, Polym. J. 2021, 57, 887-893
https://doi.org/10.1038/s41428-021-00484-0.
- Magnesium bromide (MgBr2) as a catalyst for living cationic polymerization
and ring-expansion cationic polymerization
Y. Daito, R. Kojima, N. Kusuyama, Y. Kohsaka, M. Ouchi, Polym. Chem. 2021, 12, 702-710 (DOI: 10.1039/D0PY01584A)
Collaboration with Prof. Ouchi's Group in Kyoto University; Invited for 'Molecularly Defined Polymers: Synthesis and Function'
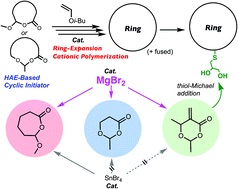
- Controls and Effects of Monomer Junctions and Sequences in Curable and
Degradable Polyarylate Containing Acrylate Moieties
Y. Kohsaka, K. Nagai, Macromol. Rapid Commun. 2021, 42, 2000570 (doi: 10.1002/marc.202000570)
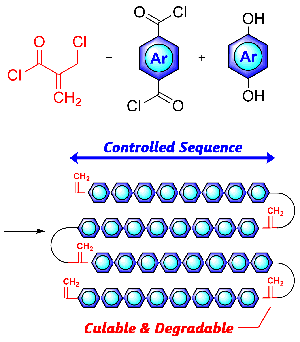
- Degradable and curable poly(conjugated ester)s prepared by acryl- and conjugate-substitutions of the ‘smallest’ monomer
Y. Kohsaka, K. Nagai, Eur. Polym. J. 2020, 141, 110049.)
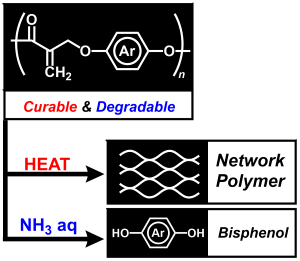
- Divergence of polycondensation by a tandem reaction based on sequential
conjugate substitutions
K. Hagiwara, Y. Kohsaka, Polym. Chemistry. 2020, 11, 5128-5132. (Back Cover)

- End-reactive poly(tetrahydrofuran) for functionalization and graft copolymer
synthesis via a conjugate substitution reaction
Y. Kohsaka, N. Nagatsuka, Polym. J. 2020, 52, 75 (doi: 10.1038/s41428-019-0258-4)
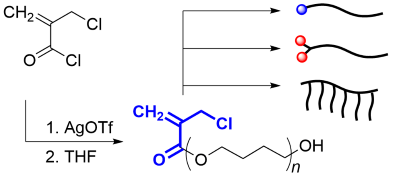
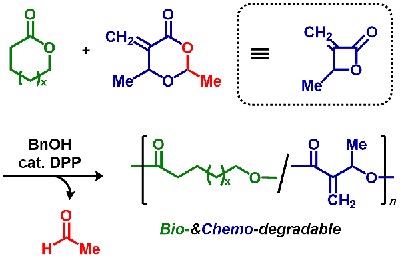
- Synthesis of Poly(Conjugated Ester)s by Ring-Opening Polymerization of
Cyclic Hemiacetal Ester Bearing Acryl Skeleton
Y. Kohsaka, M. Yamashita, Y. Matsuhashi, S. Yamashita, Eur. Polym. J. 2019, 120, 109185
- Synthesis and properties of polyethers containing 1,3-butadiene skeleton in the backbones
Y. Kohsaka, A. Hiramatsu, Chem. Lett. 2019, 48, 894.
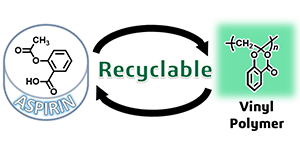
- Radical Polymerization of ‘Dehydroaspirin’ with a Formation of Hemiacetal
Ester Skeleton: A Hint for Recyclable Vinyl Polymers
A. Kazama, Y. Kohsaka, Polym. Chem. 10, 2764-2768 (2019) (10.1039/C9PY00474B) (Inside Cover).

- Conjugate substitution and addition of α-substituted acrylate: a highly
efficient, facile, convenient, and versatile approach to fabricate degradable
polymers
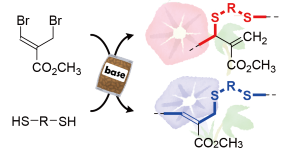
Y. Kohsaka, T. Miyazaki, K. Hagiwara, Polym. Chem. 9, 1610-1617 (2018) (DOI:10.1039/C7PY02114C). (Invited as Emerging Innovators)
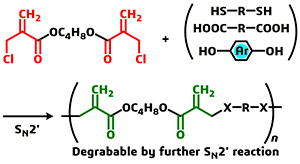
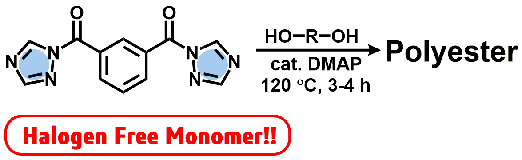
- Bifunctional Acyl-1,2,4-triazole: An Alternative Monomer of Dicarbonyl Chloride for Metal- and Halogen-Free Polyester Synthesis
Y. Kohsaka, K. Homma, I. Mori, S. Sugiyama, Y. Kimura, Chem. Lett, 47, 221-224 (2018) .
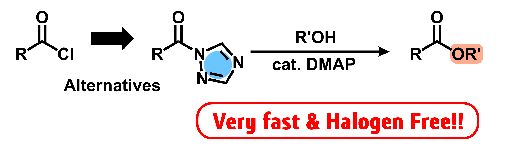
- Esterification with aromatic acyl-1,2,4-triazole catalyzed by weak base
at the rate comparable to acyl chloride
Y. Kohsaka, K. Homma, S. Sugiyama, Y. Kimura, Chem. Lett, 47, 100-102 (2018).
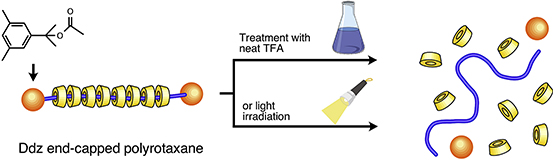
- Acid- or photo-cleavable polyrotaxane: Subdivision of supramolecular main-chain
type polyrotaxane structure induced by acidolysis or photolysis
J. Araki, Y. Honda, Y. Kohsaka, Polymer, 25, 134-137 (2017).
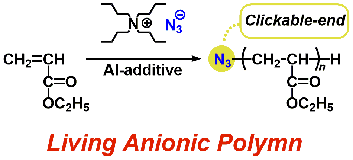
- "Anionic polymerization of ethyl acrylate initiated by tetrabutylammonium
azide: direct synthesis of end-clickable polyacrylate"
Y. Kataoka, Y. Kohsaka, T. Kitaura, S. Domae, S. Ishihara, T. Kitayama,
Polym. Chem. 8, 3858-3861 (2017). (Front Cover)
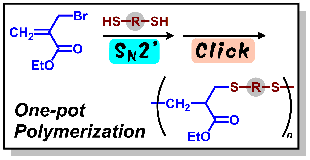
- Polymerization of α-(Halomethyl)acrylates through Sequential Nucleophilic Attack of Dithiols using a Combination of Addition–Elimination and Click Reactions
Y. Kohsaka, K. Hagiwara, K. Ito, Polym. Chem. 8, 976-979 (2017).
- Synthesis of isotactic poly[α-(hydroxymethyl)acrylate] by anionic polymerization
of the protected monomer
Y. Kohsaka, K. Yamamoto, K. Suzawa, T. Kitayama, Polym. Bull. 74, 1934-1948 (2017).
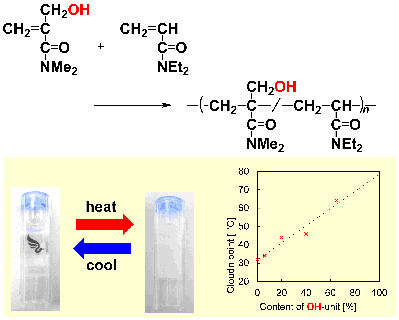
- Synthesis of thermo-responsive polymer via radical (co)polymerization of N,N-dimethyl-α-(hydroxymethyl)acrylamide with N,N-diethylacrylamide
Y. Kohsaka, Y. Tanimoto, Polymers 2016, 8, 374 (Open Access).
- Synthesis and reaction of block copolymer composed of independently clickable segments via monomer-selective living copolymerization
V. Ladelta, Y. Kohsaka, T. Kitayama, Microsystem Tech. in press.
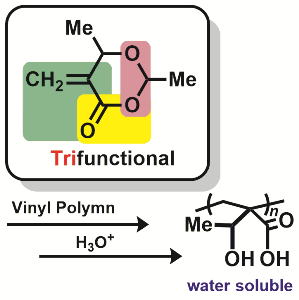
- α-Exomethylene lactone possessing acetal-ester linkage: Polymerization
and postpolymerization modification for water-soluble polymer
Y. Kohsaka, Y. Matsumoto, T. Zhang, Y. Matsuhashi, T. Kitayama, J. Polym. Sci. Part A: Polym. Chem. 54, 955-961 (2016). (Spotlight Article)
- α-(Aminomethyl)acrylate: polymerization and spontaneous post-polymerization
modification of β-amino acid ester for a pH/temperature-responsive material
Y. Kohsaka, Y. Matsumoto, T. Kitayama, Polym. Chem.6, 5026-5029 (2015).
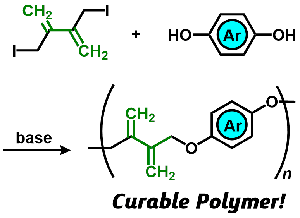
- "Termination of living anionic polymerization of butyl acrylate with
α-(chloromethyl)acrylate for end-functionalization and application to the
evaluation of monomer reactivity"
Y. Kohsaka, S. Ishihara, T. Kitayama, Macromol. Chem. Phys. 216, 1534-1539 (2015).
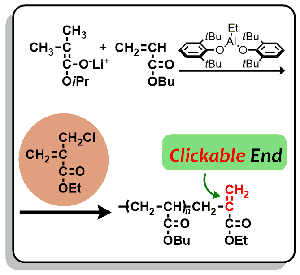
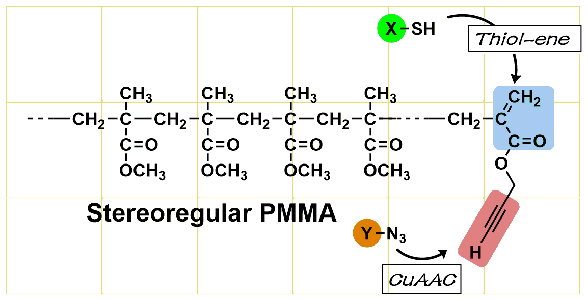
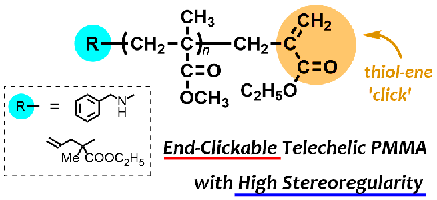
- Stereoregular poly(methyl methacrylate) with double-clickable ω-end: Synthesis and click reaction
Y. Kohsaka, K. Yamamoto, T. Kitayama, Polym. Chem. 6, 3601-3607 (2015). (Inside Cover)
- C60-Containing polymethacrylates: synthesis, properties, and potential application
as n-type semiconductor for organic solar cell
V. Ladelta, Y. Kohsaka, T. Ohsnishi, M. Matsumura, and T. Kitayama, Polym. Bull. 72, 1265-1280 (2015).
- Stereospecific Anionic Polymerization of α-(Hydroxymethyl)acrylate with Protective Group
Y. Kohsaka, K. Suzawa, T. Kitayama, Macromol. Symp. 350, 86-98 (2015).

- Synthesis and post-polymerization reaction of end-clickable stereoregular polymethacrylates via termination of stereospecific living anionic polymerization
Y. Kohsaka, T. Kurata, K. Yamamoto, S. Ishihara, T. Kitayama, Polym. Chem. 6, 1078-1087 (2015).
- Anionic alternating copolymerization of alpha-arylacrylates with methyl
methacrylate: effect of monomer sequence on fluorescence
Y. Kohsaka, E. Yamaguchi, T. Kitayama, J. Polym. Sci. Part A: Polym. Chem. 52, 2806-2814 (2014).
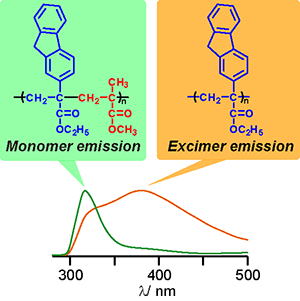
- Stimuli-degradable cross-linked polymers synthesized by radical polymerization
using a size-complementary [3]rotaxane cross-linker
K. Iijima, Y. Kohsaka, Y. Koyama, K. Nakazono, S. Uchida, S. Asai, T. Takata, Polym. J. 46, 67-72 (2014). 
- End-functional stereoregular poly(methyl methacrylate) with clickable C=C bonds: Facile synthesis and thiol?ene reaction
Y. Kohsaka, T. Kurata, T. Kitayama, Polym. Chem. 4, 5043-5047 (2013).
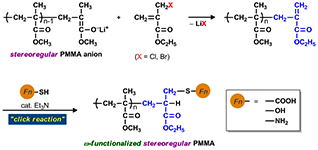
- Photo-degradable cross-linked polymer derived from a vinylic rotaxane cross-linker
possessing aromatic disulfide axle
Y. Koyama, T. Yoshii, Y. Kohsaka, T. Takata, Pure Appl. Chem. 85, 835-842 (2013). 
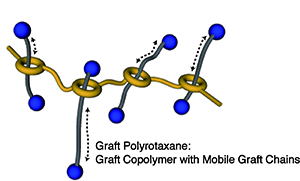
- Graft polyrotaxane: A new class of graft copolymer with mobile graft chains
Y. Kohsaka, Y. Koyama, T. Takata, Angew. Chem. Int. Ed. 50, 10417-10420 (2011). (Introduced by Chemistry World Magazine 2011)
- “Size-complementary rotaxane cross-link for stabilization and degradation
of supramolecular network”
Y. Kohsaka, K. Nakazono, Y. Koyama, S. Asai, T. Takata, Angew. Chem. Int. Ed. 50, 4872-4875 (2011). (Intoroduced by NPG Asia Materials 2011)

- An efficient synthetic entry to rotaxanes utilising reversible cleavage of aromatic disulphide bonds
T. Yoshii, Y. Kohsaka, T. Moriyama, T. Suzuki, Y. Koyama, T. Takata, Supramol. Chem. 23, 65-68 (2011). 
- Synthesis of a main chain-type polyrotaxane consisting of poly(crown ether)
and dumbbell sec-ammonium salt and its application to polyrotaxane network
Y. Kohsaka, G. Konishi, T. Takata, Polym. J. 39, 861-873 (2007).
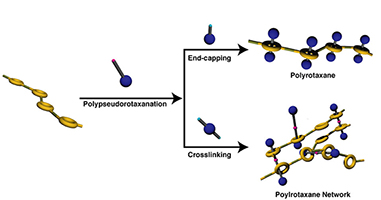
- Main chain-type polyrotaxane with controlled ratio of rotaxanated units
T. Takata, Y. Kohsaka, G. Konishi, Chem. Lett. 36, 292-293 (2007). 
|
|
| |
- 特許第7691673号 ジオール化合物ならびにその製造方法およびその重合体
▲高▼坂 泰弘, 大山 真賢, 安田 理恵, 宮内 信輔
- 特開2025-042050 環状ケテンアセタールの分解抑制方法、重合用組成物およびポリマー
▲高▼坂 泰弘, 外山 果歩
- 特開2024-160533 ポリマー
▲高▼坂 泰弘, 永沼 亘貴
- 特許第7587212号 スルフィド基を有するアリル位置換メタクリル酸エステルの製造方法
▼高▼坂 泰弘, 萩原 敬人, 塩塚 朗, 安永 篤史
- 特許第7582622号 カチオン重合開始剤、末端反応性ポリマー、末端修飾ポリマー、及びグラフトポリマー
▲高▼坂 泰弘, 長束 尚輝, 前原 賢太郎, 北村 円香
- 特開2024-155533 共重合体及びその製造方法
▲高▼坂 泰弘, 浪江 祐司, 島影 雅史, 曽根 卓男
- 特開2024-127411 重合体、粘着剤組成物、重合体粒子、及び重合体の製造方法
▲高▼坂 泰弘, 川谷 諒, 吉野 聖月
- 特開2024-124740 共重合体及びコーティング剤
▲高▼坂 泰弘, 川谷 諒, 長澤 敦, 原 脩人
- 特開2024-124741 共重合体及び光学用ハードコートフィルム
▲高▼坂 泰弘, 川谷 諒, 松井 彩香, 原 脩人, 長澤 敦
- 特開2024-124739 感光性樹脂
▲高▼坂 泰弘, 川谷 諒, 原 脩人, 長澤 敦
- 特許第7542818号 ポリ共役エステルおよびその製造方法ならびに硬化性組成物およびその硬化物
▲高▼坂 泰弘, 永井 光騎, 安田 理恵, 宮内 信輔
- 特許第7508061号 フルオレン化合物およびその製造方法ならびにその前駆体および重合体
▲高▼坂 泰弘, 大山 真賢, 安田 理恵, 宮内 信輔
- 特許第7457318号 フルオレン化合物及びその重合体並びにそれらの製造方法
▲高▼坂 泰弘, 平松 彬, 安田 理恵, 宮内 信輔
- 特許第7454176号 フルオレン化合物の製造方法並びにフルオレン化合物の用途
▲高▼坂 泰弘, 平松 彬, 安田 理恵, 宮内 信輔
- 特許第7441526号 架橋高分子化合物およびその製造方法並びに高分子化合物の生成方法
▲高▼坂 泰弘, 大矢 高史, 北河 大葵
- 特開2024-022725 不飽和ポリエステルの製造方法
▲高▼坂 泰弘, 手塚 紗英, 木村 陸人
- 特開2023-154164 連鎖移動剤及び重合体の製造方法
▲高▼坂 泰弘, 川谷 諒, 岡本 秀二, 松▲崎▼ 大典
- 特許第7368667号 α-(ハロメチル)アクリル化合物、重合体、重合体の製造方法、硬化物の製造方法及び硬化物
▲高▼坂 泰弘, 宮崎 匠
- 特開2023-124864 重合体およびその製造方法
▲高▼坂 泰弘, 大山 真賢, 安田 理恵, 鞍谷 裕嗣
- 特開2023-122572 フルオレン化合物ならびにその製造方法およびその重合体
▲高▼坂 泰弘, 大山 真賢, 安田 理恵, 鞍谷 裕嗣
- 特開2023-097771 ポリエステル樹脂とその製造方法
▲高▼坂 泰弘, 木村 陸人, 鶴見 希有
- 特許第7255799号 ポリ共役エステル及びその製造方法並びに硬化性組成物及びその硬化物
▲高▼坂 泰弘, 永井 光騎, 安田 理恵, 宮内 信輔
- 特許第7237307号 共役ジエン単位を有するポリマー及びその製造方法
▲高▼坂 泰弘, 平松 彬, 安田 理恵, 宮内 信輔
- 特開2022-172875 ジオール化合物ならびにその製造方法およびその重合体
▲高▼坂 泰弘, 大山 真賢, 安田 理恵, 宮内 信輔
- 特開2022-168399 高分子化合物の分解方法
▲高▼坂 泰弘, 田中 杏里, 萩原 敬人
- 特開2022-134520 架橋高分子化合物およびその製造方法並びに高分子化合物の生成方法
▲高▼坂 泰弘, 大矢 高史, 北河 大葵
- 特開2022-044637 α-(ハロメチル)アクリル化合物、重合体、重合体の製造方法、硬化物の製造方法及び硬化物
▲高▼坂 泰弘, 宮崎 匠
- 特開2022-042640 α位置換アクリレートオリゴマーを含有する感光性樹脂組成物及びその硬化物
▲高▼坂 泰弘, 間下 琢史, 鍔本 麻衣
- 特許第7012329号 α-(ハロメチル)アクリル化合物、重合体、重合体の製造方法、硬化物の製造方法及び硬化物
▲高▼坂 泰弘, 宮崎 匠
- 特開2021-195466 ポリ共役エステルおよびその製造方法ならびに硬化性組成物およびその硬化物
▲高▼坂 泰弘, 永井 光騎, 安田 理恵, 宮内 信輔
- 特開2021-175737 α-(置換メチル)アクリルアミド類及びその製造方法
▲高▼坂 泰弘, チンバト ニャムドルゴル
- 特開2021-175738 α-(ヒドロキシメチル)アクリル化合物およびその製造方法、ならびにその重合体
▲高▼坂 泰弘, 田中 杏里
- 特開2021-169440 スルフィド基を有するアリル位置換メタクリル酸エステルの製造方法
▼高▼坂 泰弘, 萩原 敬人, 塩塚 朗, 安永 篤史
- 特開2021-152154 両末端反応性ポリマー、多価アクリル系ポリマー、架橋多価アクリル系ポリマー、環状ポリマー、及び環状グラフトポリマー
▲高▼坂 泰弘, 長束 尚輝, 前原 賢太郎, 北村 円香
- 特開2021-127317 フルオレン化合物およびその製造方法ならびにその前駆体および重合体
▲高▼坂 泰弘, 大山 真賢, 安田 理恵, 宮内 信輔
- 特開2021-120364 フルオレン化合物の製造方法並びにフルオレン化合物の用途
▲高▼坂 泰弘, 平松 彬, 安田 理恵, 宮内 信輔
- 特開2021-120363 フルオレン化合物及びその重合体並びにそれらの製造方法
▲高▼坂 泰弘, 平松 彬, 安田 理恵, 宮内 信輔
- 特開2020-158658 ポリ共役エステル及びその製造方法並びに硬化性組成物及びその硬化物
▲高▼坂 泰弘, 永井 光騎, 安田 理恵, 宮内 信輔
- 特開2020-158659 共役ジエン単位を有するポリマー及びその製造方法
▲高▼坂 泰弘, 平松 彬, 安田 理恵, 宮内 信輔
- 特開2018-140941 α-(ハロメチル)アクリル化合物、重合体、重合体の製造方法、硬化物の製造方法及び硬化物
▲高▼坂 泰弘, 宮崎 匠
- 特許第5871223号 ロタキサン、架橋剤、架橋方法、架橋ポリマー及び架橋ポリマーの分解方法
山縣 悠介, 高田 十志和, ▲高▼坂 泰弘
- 特開2014-240482 高分子化合物
北山 辰樹, ▲高▼坂 泰弘, 大西 敏博, 小熊 潤
- 特許第5451119号 2価アルコール類、ポリカーボネート樹脂、ポリエステル樹脂、それらからなる成形体、および光学素子
高田十志和, 中薗 和子, 高坂 泰弘, 細川 勝元, 小嶋 貴博
- 特許第5213258号 ポリマーの回収方法
八子 貫之, 高坂 泰弘, 高田 十志和
- 特開2012-224559 ロタキサン、架橋剤、架橋方法、架橋ポリマー及び架橋ポリマーの分解方法
山縣 悠介, 高田 十志和, ▲高▼坂 泰弘
- 特開2010-209053 2価アルコール類、ポリカーボネート樹脂、ポリエステル樹脂、それらからなる成形体、および光学素子
高田十志和, 中薗 和子, 高坂 泰弘, 細川 勝元, 小嶋 貴博
- 特開2010-209301 ポリ擬ロタキサンの製造方法、架橋体の製造方法及びポリマーの回収方法
八子 貫之, 高坂 泰弘, 高田 十志和
|
|











